Jump to: Intro | Experts | Content | Ask CNS | Subscribe
Introduction
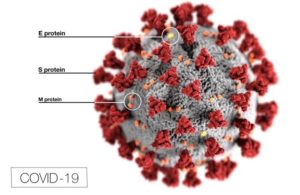
Covid-19 coronavirus diagram (Src: CDC)
Biological weapons nonproliferation and global health security are inextricably linked. From 2003-2006, I worked in Moscow, Russia, as part of a US-Russia cooperative program charged with dismantling remnants of the former Soviet offensive biological warfare (BW) program and redirecting dual-use capabilities to improve global health. In 2006, concerns that H5N1 influenza might become the next global pandemic brought the world’s attention to the neighboring country of Azerbaijan, where a cluster of human H5N1 influenza cases initially appeared to signify sustained human-to-human transmission. I moved to Baku, Azerbaijan in 2007, and spent the next four and a half years working on the same US cooperative program, this time with the government of Azerbaijan, and this time focused primarily on addressing natural threats like pandemic influenza. The implication was that reducing the risk of natural disease also reduced the risk of biological weapons, and which one was more likely? As some in my field are apt to say, Mother Nature is the world’s best bioweaponeer.
To that end, we at CNS are introducing this forum to share our perspective on COVID-19, based on decades of experience at the biosecurity/global health nexus. It in no way suggests that the ongoing pandemic is the result of a biological weapon; to the contrary, we have seen no evidence to suggest any such connection. However, we will draw lessons that can be applied to a comparable weapons context where appropriate. If nothing else, from a nonproliferation standpoint, COVID-19 has unequivocally demonstrated that we must take biological weapons and bioterrorism threats very seriously.
A word about the forum’s name: we selected World War “V” because we believe it accurately reflects our current global situation. We are, together, at war against the SARS-CoV-2 virus that causes COVID-19. In a global society, no one country is safe until we all are; persisting virus in any corner of the world may spark another wave of the pandemic and prolong our shared timeline to recovery. This challenge comes with opportunity—the opportunity to build bridges of cooperation and trust that persist long after COVID-19. We can come out of this a stronger global community—one that is united against the global health security threats of the future—or a shattered one. But rest assured, we will come out of this.
Our best wishes to you, to so many brave healthcare workers on the front lines, and to every single individual who is doing his or her part to bring this pandemic to an end the world over,
Richard Pilch, MD, MPH
Director, CNS Chemical and Biological Weapons Nonproliferation Program
CNS Experts
- Dr. Richard Pilch, Director, Chemical and Biological Weapons Nonproliferation Program (CBWNP); Senior Scientist
- Dr. Natasha Bajema, CNS Non-Resident Fellow
- Ms. Pam Berenbaum, CNS Non-Resident Fellow; Director, Global Health Program, and Professor of the Practice of Global Health, Middlebury College
- Dr. Jonathan Forman, CNS Non-Resident Fellow
- Dr. John Hart, CNS Non-Resident Fellow
- Ms. Jill Luster, CNS Senior Research Associate
- Ambassador Robert Mikulak, CNS Non-Resident Fellow
- The Honorable Andrew Weber, former Assistant Secretary of Defense for Nuclear, Chemical, and Biological Defense Programs; Senior Fellow, Council on Strategic Risks
CNS Content
- The Threat and Control of Ricin as a Weapon
 A case for accelerating ricin prevention and treatment measures.
A case for accelerating ricin prevention and treatment measures. - CNS Alumni on the COVID-19 Pandemic in Egypt and Mali
 WEBINAR: Featuring former Visiting Fellows.
WEBINAR: Featuring former Visiting Fellows. - Opinion: US Food Security During COVID-19
 Time to put our money where our mouth is.
Time to put our money where our mouth is. - The Pandemic’s Preparedness Lessons
 How the US can effectively counter biological weapons.
How the US can effectively counter biological weapons. - Biowarfare – Can It Tell Us Anything About the Corona Virus?
 PODCAST: CNS’s Dr. Pilch discusses the former Soviet Union’s biological weapons program in the context of COVID-19.
PODCAST: CNS’s Dr. Pilch discusses the former Soviet Union’s biological weapons program in the context of COVID-19. - What South Korea Did Right
 Ferenc Dalnoki-Veress on the public health policy advantages of South Korea.
Ferenc Dalnoki-Veress on the public health policy advantages of South Korea. - A Guide To Getting Serious About Bio-Lab Safety
 A four-step plan for the front lines of pandemic prevention.
A four-step plan for the front lines of pandemic prevention. - COVID-19 Update and Implications for the Future
 WEBINAR: with CNS expert Dr. Richard Pilch, BCW Nonproliferation Director.
WEBINAR: with CNS expert Dr. Richard Pilch, BCW Nonproliferation Director. - Engaging China on Bioweapons and Beyond
 Cooperating to overcome the gravest threats of nature and those of our own making.
Cooperating to overcome the gravest threats of nature and those of our own making. - Seeking Peace in a Pandemic
 A remedy for despair in a time of pandemic.
A remedy for despair in a time of pandemic.
#askcns: CNS Answers Twitter’s Pandemic Questions
Tweet a question to @JamesMartinCNS along with the hashtag #askcns and you may see your question chosen and answered here.
Please note that CNS is unable to answer all questions.
Q. What will it take – strategy, resources, et al – to prepare ourselves for the next pandemic?
I would recommend approaching this as a two-sided problem – (1) how to prevent another pandemic from starting, and (2) how to respond to a pandemic if/when it occurs:
I. Preventing another pandemic from occurring
There are really two potential sources of another pandemic like this: (1) the animal-human interface, and (2) the laboratory
1. Animal-human interface
Viruses capable of causing pandemics exist in animal reservoirs. Most major outbreaks with pandemic potential begin when humans purchase infected animals (or their meat). If we can control that interface, we can reduce the risk of sparking a pandemic. It’s another two-sided problem – supply and demand. Most approaches to control the problem focus on supply, e.g., shut down wet markets, ban the purchase of bush meat, etc., but that invariably leads to illicit and harder to control distribution networks. If we really want to solve the problem, we need to address demand as well. We have an opportunity to that end with COVID-19 – the impetus for a global shift in behavioral norms is greater than ever. I think the development of binding international standards to this end, through a regime like WHO (perhaps as an expansion of their International Health Regulations), would go a long way to reducing pandemic risk.
2. Laboratory
The SARS-CoV-2 virus that causes COVID-19 likely didn’t arise from a laboratory, but while researching its origin I learned that the US, China, and other nations are doing an extensive amount of “gain of function” research that in my opinion extends beyond scientific need such that risks outweigh potential rewards. There was a “famous” 2011 study on H5N1 influenza virus where a scientist experimentally produced a virus that retained the lethality of H5N1 while gaining the transmissibility of an H1N1-type influenza virus that spreads readily from person to person. The study sparked an international debate and much criticism, and probably should have led to a better oversight mechanism for this type of gain of function research. It didn’t, and since that time, unbeknownst to me until I did the research, scientists continue to perform such research, including on coronaviruses (see, for example, https://www.ncbi.nlm.nih.gov/pubmed/26552008, which incidentally includes scientists from the Wuhan Institute of Virology), on an alarming scale. Because a successful gain of function experiment followed by a laboratory accident could spark a pandemic, I believe it is imperative to introduce an appropriate international oversight mechanism. To me, it should include, at a minimum, two components:
- an international scientific review board that assesses proposed gain of function experiments for (a) scientific need, i.e., does the proposed research address a critical knowledge gap; (b) risk vs. benefit; and (c) roadmap for corresponding countermeasure development (e.g., in the above link, while the scientific purpose of the study – i.e., to demonstrate that a dangerous SARS-like virus can become readily transmissible from person-to-person with only minor genetic change – to me is extremely questionable, it could have led to diagnostics, therapeutics, and/or vaccine platforms that helped with COVID-19 if a technology development roadmap had been required prior to approval of the research).
- an information “clearinghouse” that limits distribution of the scientific methods and results of approved gain of function research to approved recipients only (i.e., scientific “need to know”). Whereas the open scientific literature is transparent by design, including step-by-step instructions for reproducing experiments, controlling access to approved gain of function research reduces the risk that unqualified scientists will attempt to repeat it, in unsafe laboratories, or for unsound reasons.
II. Responding to a pandemic
I’ve been working on a structured assessment of this that defines people, process, equipment/supply, and facility/infrastructure requirements, identifies gaps in these requirements, and identifies options for gap closure. It’s overly detailed, but the bottom line is that I would recommend addressing the following high-level gaps:
1. People
A Single Decision Authority
During COVID-19, it’s never been clear who is in charge. I initially expected it to be the Director of the Centers for Disease Control and Prevention (CDC), but instead Health and Human Services Secretary Alex Azar appeared to have the lead. Then it was Assistant Secretary for Preparedness and Response (ASPR) Bob Kadlec; then Vice President Pence; then the Federal Emergency Management Agency (FEMA); and sometimes the President himself. Tony Fauci at the National Institute of Allergy and Infectious Diseases (NIAID) is a strong scientific voice, but NIAID is a research institute so he is probably the wrong person, unless appointed to a dedicated czar-type post.
Healthcare Worker Surge Capacity
We either need a strategy to redeploy healthcare workers from stable areas to areas in need (e.g., North Dakota to New York City), or a Medical Reserve following a military reserve-type model where a contingent of qualified personnel maintains minimal technical readiness (e.g., training/ops for one weekend per month, or two weeks per year) and responsibility for its own equipment and supplies (e.g., personal protective equipment, testing materials and equipment, etc., including inventory control, shelf-life refresh, preventive and corrective maintenance, etc.)
2. Processes
A Comprehensive and Validated Response Plan. The first thing we always do when we develop public health systems in other countries is establish a Plan and exercise it. The Plan makes sure everyone understands who is in charge, roles and responsibilities, internal risk communication (i.e., to public health workers or government decision-makers), external risk communication (to at-risk groups and the general public), and how we will scale our response (see below). Communication is definitely key – some of the US’s early signaling during COVID-19, both internally and externally, caused confusion and put us well behind the curve in our response. And exercising the plan is the only way we can be confident that we have it right.
3. Equipment and Supplies
The key is to have an initial surge capability to hold the fort, combined with scalability to ramp up additional production.
- Personal Protective Equipment (PPE). We have PPE in the Strategic National Stockpile, but not to the initial quantities deemed necessary for surge, and apparently it may not have been refreshed based on shelf-life (I have not independently verified that point). Assuming our Response Plan includes a validated logistics approach to distribute this surge PPE based on need, the surge PPE buys us time but isn’t the answer. We then need a scalable just-in-time delivery system so that healthcare workers remain protected, households receive necessary PPE, other countries in need are supported, etc. over time. The process is to define what materials are needed to make N95 masks (for example), where we source them domestically and internationally and to what level of redundancy, what US companies can be redirected to provide these materials, what US companies can use these materials to assemble masks, and then incentivize a corresponding level of readiness akin to the medical reserve concept above (i.e., pay these companies to maintain a baseline capability that can be rapidly ramped up). Again, distribution is key, and must be a part of the Plan.
- Testing. Testing requires 3 things: (a) the test itself, (b) additional reagents and consumables necessary to perform the test; and (c) the equipment (and trained people) to perform the test.
- The test itself. Typically, we rely on CDC to make the test itself. What that involves is identifying unique genetic targets of the virus that are conserved over time, i.e., that the virus doesn’t mutate away from such that our test no longer works. It is a huge responsibility, and one that can be shared across academia and industry, whereby CDC marshals bioinformatics expertise to identify the targets, and design/production expertise to build the tests themselves. There are multiple models that could be followed to this end, for example the WHO’s collaborating centre approach.
- Reagents and consumables. The same scalability concept as PPE applies, i.e., we need a contingency plan for global supply chains (e.g., international vendors of key reagents and consumables), and we need to incentivize domestic companies capable of making reagents and consumables to maintain additional capacity (that might work against their bottom lines) in order to enable rapid ramp-up.
- Test equipment (and trained people). As with test development, performing the test requires trained people with the right equipment. The current U.S. approach utilizes clinical and commercial laboratories and public health laboratories of the CDC’s Laboratory Response Network (LRN); testing capacity can be scalably expanded to include academic, industry, and research laboratories based on need.
- Ventilators. As with PPE and testing, we need an appropriate surge of ventilators (which the Strategic National Stockpile undershot) and subsequent scalability, which was only recently initiated via the Defense Production Act. Ventilators (and ICU beds, and hospital beds in general) were a fracture point we knew we had, so it was surprising that we took so long to address the deficiency once COVID-19 was clearly coming – that scalability has to be part of the Plan, and appropriately incentivized with our industry partners.
4. Facilities/infrastructure
The general idea is to include scalable healthcare facilities and infrastructure as part of the Plan (and exercise them accordingly), whether by contracting with hotel chains, planning the use of civil infrastructure, or some alternative approach. FEMA was still identifying potential sites as COVID-19 cases were increasing exponentially, which is clearly too late; as noted above, we knew this was a fracture point, and unless hospitals are incentivized to operate at reduced capacity in the future (which would otherwise impact their bottom line) then we should expect it to remain a fracture point in need of mitigation moving forward.
—Richard Pilch, April 13, 2020
Hide Answer
Q. From @SimonRushton8: What are the pros and cons of seeing this as a ‘World War’?
We named the forum World War “V” to emphasize the global impact of COVID-19 and the need for unity in our response. Just as during World Wars I and II, nations dedicated all their resources toward the war effort; so, too, should we summon a similarly focused, whole-of-society dedication and shared sense of purpose and resolve. As stated in the introduction of the forum:
[W]e selected World War “V” because we believe it accurately reflects our current global situation. We are, together, at war against the SARS-CoV-2 virus that causes COVID-19. In a global society, no one country is safe until we all are; persisting virus in any corner of the world may spark another wave of the pandemic and prolong our shared timeline to recovery. This challenge comes with opportunity—the opportunity to build bridges of cooperation and trust that persist long after COVID-19. We can come out of this a stronger global community—one that is united against the global health security threats of the future—or a shattered one. But rest assured, we will come out of this.—Richard Pilch, April 8, 2020
Hide Answer
Q. From @EbenHarrell: Is it still the consensus that transmission of COVID is being driven through close contact? Early impressions from China was that it was being spread mostly in households. If so, does that suggest any public health interventions in the USA that might be useful?
To me, the most straightforward way to understand SARS-CoV-2 transmission is to remember that the virus primarily infects cells of the respiratory tract. The reason for this is that the virus’s S protein—which is responsible for cell binding—targets ACE2 receptors, which are prominent on cells lining the respiratory tract (among other locations). The virus reproduces inside these cells, eventually bursting the cells to release its progeny (and triggering a potentially damaging immune response). The infected individual can then spread these viral progeny in respiratory secretions that are expelled from the body by coughing, sneezing, or even singing, talking, or breathing (each of which spreads the progeny a different average distance, based on the force of expulsion).
Expelled respiratory particles range in size from quite large to very small, which dictates the likelihood and route of further infection as follows:
- The largest particles fall out of the air quite quickly, settling on surfaces where they can survive from hours to days (depending on the surface; external conditions like UV light, temperature, and humidity; and other factors). If another person touches the surviving virus and then touches his or her mouth, nose, or eyes, infection can result. This is called “contact transmission.”
- Smaller but still relatively large particles in the 5-10 micron range also fall out of the air, but they travel further through the air before they do. If they directly contact another person’s mouth, nose, or eyes (or if another person inhales them directly), infection can result. As a general rule of thumb, this size particle is expected to fall out of the air within 2 meters (~6 feet), which is why social distancing guidelines recommend that individuals maintain a distance of 6 feet. This is called “respiratory droplet” or simply “droplet transmission.”
- Smaller-sized particles which can potentially travel further distances for longer durations do not appear to readily transmit SARS-CoV-2. This would be called “aerosol” or “airborne transmission;” biological agents that can spread by this route, such as the measles virus, typically infect a higher number of contacts per case (called the R0 value) than SARS-CoV-2.
The CDC and WHO are in agreement that airborne transmission of SARS-CoV-2 is unlikely outside the medical setting, where aerosol-generating procedures may be performed. The CDC and WHO also agree that we are still learning about how COVID-19 spreads. However, while the CDC considers respiratory droplets to be the primary route of transmission, the WHO appears to be reserving judgment, instead citing both respiratory droplet and contact transmission as primary routes.
Whether person-to-person transmission occurs via respiratory droplets or contact, it’s clear that close proximity increases risk, which is why we see higher rates of infection in the healthcare setting (despite careful precautions) and, as you note, in households. China mitigated the risk of household transmission by establishing mandatory “quarantine hospitals” for infected family members, which reduced the risk of transmission to other family members. For the US to adopt such a policy, we would first need an adequate testing capability to identify infected individuals; then the ability to safely relocate infected individuals to care facilities that have been designated and prepared for this purpose; and, perhaps most importantly, the resolve of various levels of government to enact such a policy, and the will of the people to comply.
In the absence of quarantine hospitals, family members can limit unnecessary activities outside the home; practice good hand hygiene and disinfect items brought into the home; self-isolate if feeling sick; and take special precautions when interacting with high-risk individuals in the home, such as elderly family members with underlying health conditions, through physical distancing, glove/mask use, or other measures.
A useful reference for the latest COVID-19 scientific data, including our understanding of routes of transmission, is: https://www.dhs.gov/publication/st-master-question-list-covid-19
—Richard Pilch, April 8, 2020
Hide Answer
Q. Do we know for sure if individuals who are asymptomatic also have the same COVID-19 antibodies as those who display symptoms?
We would expect to see the same protective antibodies whether a person has limited symptoms or very bad symptoms. There are two overlapping antibody curves – early in the disease course, we see a rise in IgM antibodies, which subsequently decline as IgG antibody production accelerates and the disease resolves. IgG antibodies then decline over time, but some remain as our “memory” that provides some level of longer term protection. We don’t know how long that memory lasts, but it should be enough to at least get us safely onto an annual vaccination schedule; studies on SARS (2003) found that IgG antibodies provided protection for approximately 3 years, and early animal models of COVID-19 support their protective effect against reinfection.
These IgG antibodies are also the basis for using convalescent plasma of SARS-CoV-2 survivors to treat infection. On April 3, 2020, the U.S. Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) for this application.
What we’re desperate for are serologic tests looking for these antibodies, so we can know (1) when people are recovered; (2) how long protection lasts; and, importantly, (3) how much of the general population has been exposed. These serological tests are different from the PCR tests that use a nasopharyngeal swab to identify viral nucleic acid; these require a blood sample, and can be done using relatively inexpensive lateral flow assays (similar to a pregnancy test, for example) or using a machine called an ELISA machine. The FDA issued its first EUA for a serologic test on April 1, 2020.
Once the test becomes available, I would recommend we use this test on recovered patients first; then healthcare workers to determine levels of exposure; and then key sentinel populations that can give us a sense of underlying exposure in corresponding regions. For example Transportation Security Administration (TSA) workers at airports; bus drivers; grocery store and pharmacy workers; etc. That level of knowledge would truly be game-changing.
—Richard Pilch, April 8, 2020
Hide Answer
Q. I would be interested in your take on the possibility of this being an accidental release from Wuhan labs. I’ve seen a couple of reasonable takes on this in Bulletin and elsewhere but am curious as to what your group thinks of the possibility that this was not a purely natural event. Not that they were manufacturing weapons but they were doing lab tests with existing or altered viruses (One of the Chinese virologists at Wuhan appears to have participated in studies including at UNC of particular interest) to produce vaccines and that the virus leaked.
The bottom line is that from strictly a molecular epidemiology standpoint, we cannot rule out a laboratory origin.
There are two key genetic differences between SARS-CoV-2 and its nearest neighbor (a bat coronavirus that shares ~96% of its genome). Both of these differences are in SARS-CoV-2’s genetic code for the virus’ spike (“S”) protein, which is responsible for cell binding.
The first difference involves the S protein’s receptor binding domain (RBD) – the RBD of SARS-CoV-2 resembles the RBD of a pangolin coronavirus. SARS-CoV-2 and the pangolin coronavirus are dissimilar otherwise. While only a hypothesis, the explanation may be that a pangolin was infected by both the bat coronavirus and the pangolin coronavirus at the same time, and those viruses exchanged parts to give us the S protein of the pangolin coronavirus on what is otherwise the bat coronavirus. This change likely improves binding to the ACE2 receptor in the human respiratory tract, and is consistent with our understanding of how genetic shifts happen in nature.
What is less clear is the second difference in the S protein’s genetic code. This difference involves a small genetic sequence insertion coding for a “polybasic cleavage site” that may enhance SARS-CoV-2’s ability to cause infection. We have seen similar insertions occur naturally in bat coronaviruses, suggesting that it may be a common natural evolutionary process, but we haven’t seen this specific insertion in a virus closely related to SARS-CoV-2. The laboratory origin argument therefore holds that scientists may have been conducting passaging experiments to see if the virus can evolve the ability to spread efficiently from person-to-person, resulting in this mutation. A subsequent laboratory release (whether via a needlestick, animal bite, disposal of inadequately decontaminated infections waste, etc.) would then have the potential to spark an outbreak/pandemic.
In my opinion, far too much of this type of “gain of function” research goes on, including – as you note – on coronaviruses in both the U.S. and at the Wuhan Institute of Virology (see, for example, https://www.ncbi.nlm.nih.gov/pubmed/26552008).
Here are select references on the molecular epidemiology of SARS-CoV-2 that informed our analysis:
https://www.nature.com/articles/s41586-020-2012-7
https://www.biorxiv.org/content/10.1101/2020.02.17.951335v1
https://www.nature.com/articles/d41586-020-00548-w
https://www.nature.com/articles/s41591-020-0820-9
https://www.sciencedirect.com/science/article/pii/S0092867420303287—Richard Pilch, April 8, 2020
Hide Answer
Q. From @GeoffreyNicole1: It doesn’t matter the surface (or maybe it does?), but I am a covid-19 organism sitting on a glove, a carton, my finger, a dollar bill…am I alive for 20 minutes? Two hours? How long can I infect someone who touches the surface I am on?
We’re still learning about SARS-CoV-2 stability in the environment, but experimental studies suggest that the virus can survive for hours to days depending on the surface. The key differentiator in these studies is whether the surface is porous (e.g., cardboard, paper) or nonporous (e.g., metal, plastic). Virus detection is comparatively limited on porous surfaces, but it is unclear whether that is because the virus doesn’t survive as well on these surfaces or whether it is simply harder to recover the virus from porous versus non-porous surfaces. There are also some exceptions to this generalization – the virus has been recovered from surgical masks after 7 days, [1] whereas the virus does not appear to survive well on copper.
A recent study of SARS-CoV-2 stability on various surfaces demonstrated the following: [2]
- Copper – up to 4 hours (no viable virus detected after that duration)
- Cardboard – up to 24 hours (no viable virus detected after that duration)
- Stainless steel – up to 72 hours, but with considerable degradation over time
- Plastic – up to 72 hours, but with considerable degradation over time
Experts at the Department of Homeland Security (DHS) Science & Technology Directorate are working with DHS’s national laboratory, the National Biodefense Analysis and Countermeasures Center (NBACC), to better understand this and other key knowledge gaps; their master question list, which includes a section on environmental stability.
Notes:
[1] https://www.sciencedirect.com/science/article/pii/S2666524720300033
[2] https://www.nejm.org/doi/full/10.1056/NEJMc2004973
—Richard Pilch, April 17, 2020
Hide Answer
Q. What is the latest thinking about immunity for those who have already had COVID-19 and fully recovered? How much hope should we have for plasma transfusions from recovered patients into sick patients?
Many experts reasonably expect that individuals who are infected and recover from COVID-19 will have some level of immunity to the virus post-infection. However, because COVID-19 is a new virus, we lack sufficient data to understand the level of protection such immunity might provide, and how long such immunity might last. Comparative analysis of SARS-CoV (2003) in humans as well as SARS-CoV-2 in non-human primates suggest that immunity should be enough to at least get us safely onto an annual vaccination schedule; studies on SARS (2003) found that IgG antibodies provided protection for approximately 3 years [1], and early animal models of COVID-19 support their protective effect against reinfection. [2]
We similarly lack sufficient data to understand the prospects for plasma transfusions from recovered patients into sick patients (“convalescent plasma therapy”), but the concept is not new [3] and early evidence appears promising. Two published studies on a very small number of COVID-19 patients in China found that the treatment was generally well-tolerated, reduces viral load, and may lead to improved clinical outcomes. [4] These early studies should be interpreted with caution, however, due to several limitations including the very small sample of patient treated (five and 10, respectively), their selection as a non-random patient sample, and the range of additional intervention measures that were used. More rigorous clinical trials are needed – and are now underway – to establish both the risks and benefits of using convalescent plasma therapy for patients with COVID-19. [5]
In the interim, efforts to reap the potential benefits of convalescent plasma therapy are moving rapidly. Scientists and doctors from multiple organizations have formed the National COVID-19 Convalescent Plasma Project, providing information for health care providers, donors, and patients. [6] On March 24, 2020, the U.S. Food and Drug Administration (FDA) announced that it would begin to facilitate access to convalescent plasma from people who have recovered from the COVID-19 virus. [7] On April 3, 2020, the FDA issued Emergency Use Authorization (EUA) for this application and named the Mayo Clinic as the lead institution for an Expanded Access Program, “providing coordinated access to investigational convalescent plasma for hospitalized patients with severe or life-threatening COVID-19, or those at high risk of progression to severe or life-threatening disease.” [8] As of April 19, 2020, 2040 patients are enrolled in the program and 600 have received transfusions.
Notes:
[1] See for example: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851497/
[2] See for example: https://www.the-scientist.com/news-opinion/monkeys-develop-protective-antibodies-to-sars-cov-2-67281
[3] See for example: https://www.jci.org/articles/view/138003; https://hub.jhu.edu/2020/03/13/covid-19-antibody-sera-arturo-casadevall/; https://www.jci.org/articles/view/138745/pdf
[4] See for example: https://jamanetwork.com/journals/jama/fullarticle/2763983; https://jamanetwork.com/journals/jama/fullarticle/2763982
[5] See for example: https://clinicaltrials.gov/ct2/show/NCT04344015?type=Intr&cond=COVID-19&draw=3&rank=17 ; https://clinicaltrials.gov/ct2/show/NCT04342182; https://hub.jhu.edu/2020/04/03/blood-plasma-sera-covid-19-fda-approval/; https://redcap.uchicago.edu/surveys/index.php?s=X997ND9N77; https://www.uchicagomedicine.org/forefront/coronavirus-disease-covid-19/clinical-trial-to-explore-convalescent-plasma-transfusions-for-covid-19-patients; https://recruit.cumc.columbia.edu/clinical_trial/1929
[6] See for example: https://ccpp19.org/index.html; https://ccpp19.org/healthcare_providers/index.html
[7] See for example: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-24-2020; https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma; https://www.fda.gov/media/136798/download
[8] See for example: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma; https://www.uscovidplasma.org/—Jill Luster, April 20, 2020
Hide Answer
